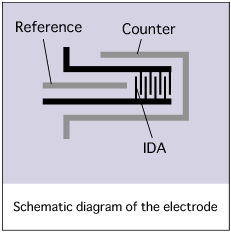
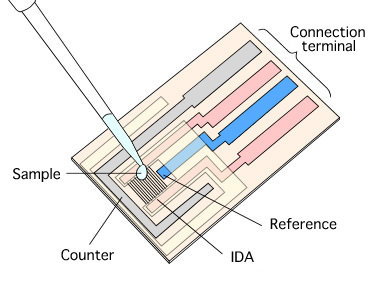
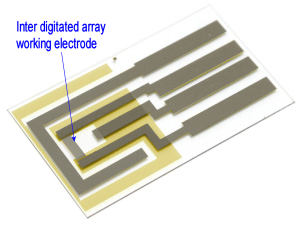
CV measurement using Interdigitated Array Electrodes
IDA Electrode is a pair of band electrode combined and meshes with each other as a generator electrode and collector electrode, therefore it is possible to make an electrochemical redox cycle upon the electrode as showed in figure.
By occurring the redox cycle on electrode increasing electrolysis current to raise measurement sensitivity. In experiment using common electrodes to analyze a small quantity of sample solution, the sample will consumed and exhausted due to electrolysis. However using this Interdigitated array electrode, the oxidation-reduction reaction occur repeatedly so the sample solution will not exhausted.
 |
 |
keyword is diffusion and concentration slope
Substance to be oxidized/reduced into the sample solution is transported by diffusion to the electrode surface. Diffusion is the physical phenomenon of the molecular species transport from high to low concentrated region.
Interdigitated 65 pairs of generator/collector electrodes set off electrochemical RedOx cycling continuously as shown in Fig.1. This reaction significantly boosts sensitivity of the electrode. Furthermore, samples are preserved when Dual (Red-Ox) Mode is chosen - Not like as Single Mode eats up samples at measurements.
In single mode, the decrease of current response become very small because of the samples substance consumption due to the electrolysis. |
 |
| |
| Compared with single mode, dual mode measurement has increased in 30 times the current value. |
 
 Zoom Zoom |
| |
The voltammograms of ferrocene samples ((a),(c): 10 µL , (b),(d): 0.2 µL) using IDA electrode ((a),(b): Dual mode; (c),(d): Single Mode) are shown below. As they indicate, measuring mode makes obvious difference on their responses. Dual mode (a),(b) reinforce reduction current at collector electrodes with increase of oxidation current at generator electrodes, in addition, the current value is not affected by the sample volume, only depends on the concentration of the sample. At the measurement (d), comparing with (c), the current response is scarcely obtained, because the sample was consumed during the experiment, indicating that electrode response current of single mode is greatly dependent on the amount of sample solution.
Size
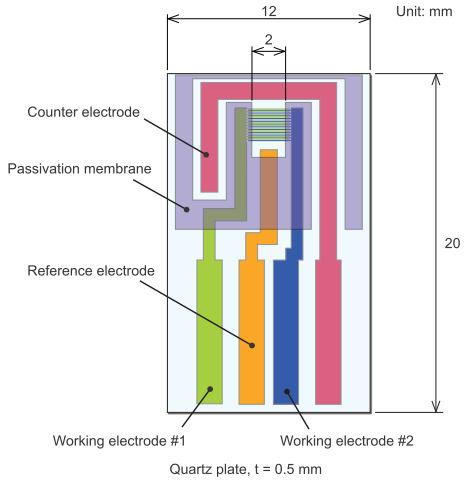 |
Substrate Dimension |
| Width |
12.0±0.1 mm |
| Length |
20.0±0.1 mm |
| Thickness |
0.5 mm |
| Electrode Thickness |
| Au |
90 nm |
| Pt |
| C |
1.2 ± 0.1 μm |
| ITO |
100 nm |
| Adhesive layer - Passivation membrane thickness |
| Ti |
approximately 10 nm
(∗only for Au and Pt) |
| Passivation membrane |
about 1 μm |
IDA electrode expanded diagram
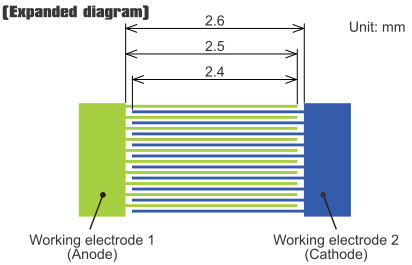
| Catalog No. |
Description |
Width (µm) |
Interval (µm) |
Length (mm) |
Number of feet (pairs) |
Film thickness |
| 012125 |
IDA electrode (Au) |
10 |
5 |
2 |
65 |
90 nm ∗ |
| 012126 |
IDA electrode (Pt) |
10 |
5 |
2 |
65 |
90 nm ∗ |
| 012127 |
IDA electrode (Carbon) |
10 |
5 |
2 |
65 |
1.2 +/- 0.1 µm |
| 012128 |
IDA electrode (ITO) |
10 |
5 |
2 |
65 |
100 +/- 20 nm |
| 012129 |
IDA electrode (Au) |
3 |
3 |
2 |
65 |
90 nm ∗ |
| 012130 |
IDA electrode (Pt) |
3 |
3 |
2 |
65 |
90 nm ∗ |
| 012257 |
IDA electrode (Au) |
2 |
2 |
2 |
65 |
90 nm ∗ |
| 012258 |
IDA electrode (Pt) |
2 |
2 |
2 |
65 |
90 nm ∗ |
| w/o passivation membrane |
| 012259 |
IDA electrode (Au) without passivation membrane |
10 |
5 |
2.5 |
65 |
90 nm ∗ |
| 012262 |
IDA electrode (Pt) without passivation membrane |
10 |
5 |
2.5 |
65 |
90 nm ∗ |
| 012266 |
IDA electrode (Carbon) without passivation membrane |
10 |
5 |
2.5 |
65 |
1.2 +/- 0.1 µm |
| 012265 |
IDA electrode (ITO) without passivation membrane |
10 |
5 |
2.5 |
65 |
100 +/- 20 nm |
| 012260 |
IDA electrode (Au) without passivation membrane |
3 |
3 |
2.5 |
65 |
90 nm ∗ |
| 012263 |
IDA electrode (Pt) without passivation membrane |
3 |
3 |
2.5 |
65 |
90 nm ∗ |
| 012261 |
IDA electrode (Au) without passivation membrane |
2 |
2 |
2.5 |
65 |
90 nm ∗ |
| 012264 |
IDA electrode (Pt) without passivation membrane |
2 |
2 |
2.5 |
65 |
90 nm ∗ |
| OPTION |
| 011066 |
Cable kit for IDA electrode |
| 011464 |
Ag/AgCl Ink for reference electrode (2.0 mL) |
∗ For Au and Pt, the thickness of the titanium adhesive layer is about 10 nm, resulting in a total thickness of 100 nm.
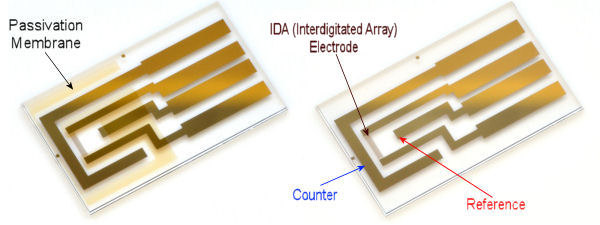
The handling of until measurement
For the high-sensitivity detection by redox cycle using IDA electrode, dual potentiostat is required.
In addition, CS-3A Cell stand to reduce the noise, and the coating of the reference electrode portion to have the stable reference potential, applying such as silver-silver chloride ink, are recommended.
Handling precautions of IDA electrode
The IDA electrode is a very sensitive product. Here, some advice for the preservation and handling of IDA electrode will be described.
Attention for receiving the product
After receiving the product, it is recommended to test the insulation between the working electrodes using a tester.
Attention for opening the package
- When you will take out the electrode from the package, catch with the fingers at the edge of the glass substrate or pinch the glass plate carefully with tweezers. Glass substrate could break easily, so keep away from excessive force and strain.
- Do not touch the pattern area of the electrode directly. If you touch the pattern area, it could be peeled or break out.
Attention for cleaning before use
- Do not clean in ultrasonic cleaner.
- Do not clean the electrode with strong acid or base solution.
- Do not clean in ozone cleaner.
- Do not scratch the electrode surface to avoid the pattern area conductivity break down.
- If you want to clean the electrode with organic solvent, only rinse with acetone or ethanol. Do not immerse the electrode in an organic solvent for long time to avoid peeling of the electrode pattern and passivation membrane.
Attention in measurement
- The electrode can not be used in high and low temperature.
- Do not use the electrode in strong acid or base solution.
- Do not apply excessive oxidation or reduction potential.
- Physical or chemical modification of the electrode will be for your own responsibility. We do not guarantee the electrode characteristics after modification.
- This electrode is assumed as disposable. Reuse of the electrode is not recommended.
Attention in storage
If long-term storage, put the electrode in the case. Store the case in clean space such as desiccator, away from heat and moisture.
CAUTION
Any defects in the appearance (ex: pinhole on lead area, burr of glass) without any influence to the measurement, are not covered under warranty.
Technical Note
Experimental performance of the IDA electrode, with a small width (3 µm and 2 µm) is compared with the 10 um width IDA electrode.
Also, the collection efficiency was compared for the three types of the IDA electrodes.More fine IDA (Pt/Au) Electrode has been developed. Its 3 µm pitch working electrodes significantly increase redox cycle and allow researchers to perform ultra-sensitive measurement.
IDA Electrode 3 µm
More fine IDA (Pt/Au) Electrode has been developed. Its 3 µm pitch working electrodes significantly increase redox cycle and allow researchers to perform ultra-sensitive measurement.
IDA Au (3 µm) IDA Pt (3 µm)

We recommend to apply Ag/AgCl ink to a reference electrode in order to stabilize potential of the electrode.
Technical data concerning IDA Electrode 3 µm
|
 Fig. 1 - Accuracy Fig. 1 - Accuracy
|
A CV curve obtained by 3 µm IDA Electrode Au [A] mirrored that of 3 µm IDA Electrode Au [B], which means both of the IDA Electrodes were produced very accurately.
- Left: 3 µm IDA Electrode Au [A]; Right: 3 µm IDA Electrode Au [B]
- Sample: ferrocene methanol (1 mM/ 0.5 M NaCl solution)
- Mode: Single
- Sweep Rate: 30 mV/s
|

Fig. 2 - CV by Dual mode
|
- Mode: Dual
- Sweep Rate at G-Electrodes: 10 mV/s
- Applied Voltage to C-Electrodes: -0.2 V
|
Fig. 3 & 4 - Comparison of 3 µm IDA Au with 10 µm by Dual Mode
For 3 µm electrode, the limiting current of ferrocene methanol by CV Dual mode was 8 times as much as of the peak current by Single mode, while this ratio for 10 µm electrode was about 4. This means large increase of the redox cycle.
 
Figure 3.
IDA Electrode: 3 µm Au
Sweep Rate: 10 mV/s |
 
Figure 4.
IDA Electrode: 10 µm Au
Sweep Rate: 10 mV/s |
| *Larger area in 10 µm IDA electrode gives the appearance as if its absolute current is larger than 3 µm of IDA |
|
 
Fig. 5 - Capture Efficiency
|
Figure 5 shows a result of LSV Dual mode measurement. Its collection efficiency was proved to be 96.4% by our calculation from limiting current of Collector electrodes and Generator electrodes.
- Method: LSV (Linear Sweep Voltammetry)
- Mode: Dual
- Sample: ferrocene methanol (1 mM/0.5 M NaCl solution)
- Sweep Rate: 10 mV/s
|
Besides CV and LSV electrochemical techniques, our IDA electrode is available for various electrochemical measuring techniques such as chronoamperometry, normal pulse voltammetry, differential pulse voltammetry etc.
Researchers can obtain more insights by using IDA electrodes.
IDA Electrode 2 µm
More fine IDA (Pt/Au) Electrode has been developed. Its 2 µm pitch working electrodes significantly increase redox cycle and allow researchers to perform high-ultra-sensitive measurement.
Technical data concerning IDA Electrode 2 µm
A CV curve obtained by 2 µm IDA Electrode Au.
CV by Single mode

- Sample: 1 mM ferrocene methanol (0.5 M NaCl solution)
- Mode: Single
- Sweep Rate: 10 mV/s
CV by Dual mode

- Sample: 1 mM ferrocene methanol (0.5 M NaCl solution)
- Mode: Dual
- Sweep Rate at G-Electrodes: 10 mV/s
- Applied Voltage to C-Electrodes: -0.05 V
Its collection efficiency was proved to be 97% by our calculation from limiting current of Collector electrodes and Generator electrodes.
Capture Efficiency
Compared with IDA Au 10 µm and 3 µm, the capture efficiency of the 2 µm is higher, 93% for 10 µm and 95% for 3 µm.
For 2 µm electrode, the limiting current of ferrocene methanol by CV Dual mode was 11 times as much as of the peak current by Single mode, while this ratio for 10 µm and 3 µm electrode were about 4 and 7.5, respectively. This means large increase of the redox cycle.